Photo credits: ScenTree SAS
General Presentation
-
CAS N° : 20407-84-5
-
EINECS number : 243-797-2
-
FEMA number : 2402
-
Density : 0,841 - 0,849 @25°C
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Head/Heart
-
Price Range : €€€
-
Appearance : Pale yellow liquid
-
FLAVIS number : 05.144
-
JECFA number : 1350
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 126°C
-
Detection Threshold : Donnée indisponible.
-
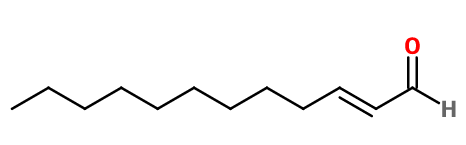
Molecular formula : C12H22O
-
Log P : 4,53
-
Molecular Weight : 182,31 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 113°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
comparing it to other aldehydesas Aldehyde C-12 lauric, Mandarin Aldehyde has a more fuity smell of Mandarin Yellow EO.
Stability :
Aldehydes may form diethylacetals in alcoholic perfumes, with no real impact on their smell
Uses in perfumery :
Mandarin Aldehyde is used for all types of perfumes, for fresh notes. Used in floral-aldehydic, coniferous, ambery notes, for a contribution of a characteristic aldehydic note. Brings freshness, boosts the head and gives tenacity to citrus notes.
Year of discovery :
Data not available.
Isomerism :
Mandarin Aldehyde has two diastereoisomers (Z) and (E). The isomer (E) in most often used in perfumery, to the detriment of the isomer (Z). Concentration of trans isomer is minimum 93%
Synthesis precursor :
Mandarin Aldehyde forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Natural availability :
Mandarin Aldehyde can be extracted from Coriander Leaf EO in its natural state, where it is present at about 20%.
Synthesis route :
Mandarin Aldehyde is an aliphatic aldehyde. As other aldehydes, it can be synthesized by reacting dodecenyl halides (chloride for example) with dimethyl sulfoxide (DMSO), followed by an alkaline treatment with sodium bicarbonate. Other synthesis routes exist using a Rosenmund reaction from dodecenic acid and an acid chloride.
Regulations & IFRA
This ingredient is not restricted

