Photo credits: ScenTree SAS
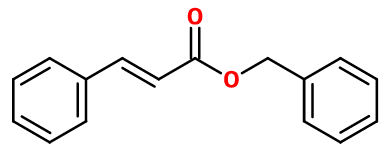
Benzyl cinnamate
Benzyl 3-Phenylprop-2-enoate ; Benzyl 3-phenyl propenoate ; Benzyl alcohol cinnamate ; Benzyl gamma-phenyl acrylate ; Benzyl-3-phenyl propenoate ; Cinnamein ; Phenyl methyl 3-phenyl-2-propenoate ; Phenylmethyl 3-phenylprop-2-enoate

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 103-41-3
-
EINECS number : 203-109-3
-
FEMA number : 2142
-
Density : 1,1
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Heart/Base
-
Price Range : €€
-
Appearance : White to pale yellow solid
-
FLAVIS number : 09.738
-
JECFA number : 670
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 350°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C16H14O2
-
Log P : 4,1
-
Molecular Weight : 238,29 g/mol
-
Fusion Point : 34°C
-
Flash Point : 180°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
Benzyl Cinnamate is one of the 26 allergens in perfumery.
Stability :
May form Cinnamic Acid through time.
Tends to get a yellow color through time.
Uses in perfumery :
Benzyl Cinnamate is used mainly in oriental perfumes as a fixative note. Used in heavy fragrances, reproducing notes of vanillic tree resin.
Year of discovery :
Data not available.
Isomerism :
Benzyl Cinnamate has a double bond allowing the existence of two diastereoisomers (Z) and (E) which have a similar smell.
Synthesis precursor :
Benzyl CInnamate is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Benzyl Cinnamate is found in Peru Balsam Resinoid and in Tolu Balsam Resinoid as well as in Copahu EO or Cabreuva EO, among others. Therefore, natural Benzyl Cinnamate can be extracted from these balsams and essential oils.
Synthesis route :
Benzyl Cinnamate is synthesized by an esterification reaction, using Cinnamic Acid, Benzyl Alcohol and an acidic catalyst such as sulfuric acid.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,36 % 0,11 % 1,2 % 2 % 0,51 % 0,51 % 0,51 % 0,17 %1,2 % Cat.5A B C DCat.6 0,51 % 0,51 % 0,51 % 0,17 %1,2 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 2,4 % 2,4 %0,17 % 3,9 % 3,9 % 14 %0,17 % 0,17 %No Restriction Cat.10A BCat.11A BCat.12 3,9 % 14 %0,17 % 0,17 %No Restriction

