Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
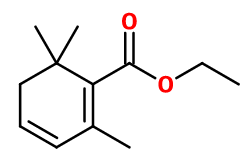
CAS N° : 35044-59-8
-
EINECS number : 252-335-9
-
FEMA number : Donnée indisponible.
-
Density : 0,967
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Head/Heart
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 239°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C12H18O2
-
Log P : Donnée indisponible.
-
Molecular Weight : 194,27 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 85°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
Safranal has a smell quite similar to Ethyl Saranate, both used for their spicy notes of saffron and fruity notes. Nevertheless, the synthesis of Ethyl Safranate is less expensive and is preferred to Safranal.
Stability :
Esters may form acetic acid through time. Ethyl Safranate still is globally stable in perfumes and diverse functional bases, except acid cleaners and very alkaline products.
Uses in perfumery :
Ethyl Safranate is used in saffron notes, fruity notes of yellow fruits such as plum and other stone fruits, for notes of cooked apple, in woody perfumes to bring a fruity note and in floral notes. It can be used in various alkaline bases for its stability.
Year of discovery :
1955
Isomerism :
The Ethyl Safranate most commonly used in perfumery is a mixture of alpha, beta and gamma isomers of the molecule. These isomers are not isolated for their use in perfumery. In addition, Ethyl Safranate is a constitutional isomer of Scentenal® and Hyacinth Body®. Their smell is however very different.
Synthesis precursor :
Ethyl Safranate does not interfere with the synthesis of another compound of olfactory interest.
Natural availability :
Ethyl Safranate is not available in its natural state.
Synthesis route :
Ethyl Safranate can be synthesized by a reaction between Mesityl Oxide and Ethyl Acetoacetate. Both will cyclize when put in an acid medium. The obtained intermediate molecule can be reduced and dehydrated to obtain Ethyl Safranate.
Regulations & IFRA
This ingredient is not restricted

