Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 28371-99-5
-
EINECS number : 248-995-2
-
FEMA number : Donnée indisponible.
-
Density : 0,97
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Base
-
Price Range : €€€
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
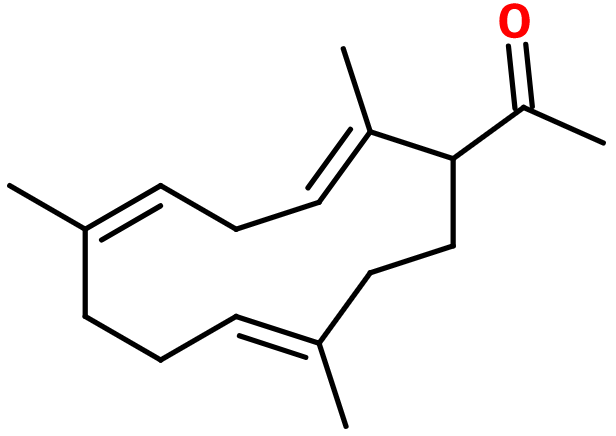
Molecular formula : C17H26O
-
Log P : Donnée indisponible.
-
Molecular Weight : 246,39 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : >93°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
In the woody-ambery family, Trimofix® has a distinctive very dry note of sawdust, as Karanal® and Cedramber®.
Stability :
Stable in perfumes and various functionnal bases.
Uses in perfumery :
Trimofix® is used in woody notes and in male perfumes, to bring woody, ambery and dry power. It can be overdosed as it is sweeter than other woody-ambery materials.
Year of discovery :
1969
Isomerism :
Trimofix® has three double bonds. Their conformation does not matter for this compound. It is imposed during the synthesis and no isomer is favoured. Trimofix® also is a constitutional isomer of Vertofix®, having a distinctive cedarwood smell.
Synthesis precursor :
Trimofix® is not a precursor for the synthesis of another material used in perfumery.
Natural availability :
Trimofix® is not reported as found in nature, and can thus not be extracted from any plant.
Synthesis route :
Synthesis of Trimofix® can be done starting from its corresponding trimethylcyclododecatriene, thanks to an acetylation reaction using acetic anhydride, in the presence of boron trifluoride diethyl ether.
Regulations & IFRA
-
IFRA 51th : This ingredient is restricted by IFRA
-
Restriction type : RESTRICTION
-
Cause of restriction : DERMAL SENSITIZATION AND SYSTEMIC TOXICITY
-
Amendment : 49
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,00016 % 0,13 % 0,4 % 2,4 % 0,6 % 0,52 % 0,6 % 0,17 %0,00016 % Cat.5A B C DCat.6 0,6 % 0,52 % 0,6 % 0,17 %0,00016 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,87 % 0,87 %0,17 % 2,2 % 2,2 % 4,4 %0,17 % 0,17 %No Restriction Cat.10A BCat.11A BCat.12 2,2 % 4,4 %0,17 % 0,17 %No Restriction

