Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
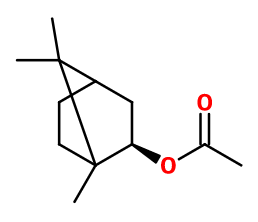
General Presentation
-
CAS N° : 125-12-2
-
EINECS number : 204-727-6
-
FEMA number : 2160
-
Density : 0,983
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Head
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 09.218
-
JECFA number : 1388
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 231°C
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C12H20O2
-
Log P : 3,86
-
Molecular Weight : 196,29 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 89°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
The smell of Isobornyl acetate is close to natural pines, as it is a blend of the facets we can find in these extracts : from the balsamic facet of Scots Pine Absolute to the camphorated facet of Siberian Pine EO. It remains more artificial and foody.
Stability :
acetates may form acetic acid through time
Uses in perfumery :
Isobornyl acetate is used in fougere, woody, red fruits notes to give a blackcurrant effect. Useful in incense notes. Used in all types of perfumery, but especially in detergents.
Year of discovery :
Data not available.
Isomerism :
Isobornyl acetate contains an asymmetric carbon. However, it is still the racemic mixture that is used in perfumery. Geranyl acetate, Neryl acetate, Linalyl acetate and Terpenyl acetate are isomers of Isobornyl acetate. However, the first two are closer to pear and rose, while the other two are reminiscent of Bergamot EO.
Synthesis precursor :
Isobornyl acetate is used as an intermediate to the synthesis of Camphor.
Natural availability :
Isobornyl acetate is present in every natural raw material that contains Bornyl acetate (Scots Pine EO among others). Thus, it can easily be extracted in its natural state.
Synthesis route :
Isobornyl acetate is an ester synthesized in two possible ways: either by esterification of acetic acid or acetic anhydride with Isoborneol, in the presence of an acid catalyst, or by treatment of Camphene in an acid medium that contains acetic acid. Following this process, a racemic mixture of the two isomers of this ester is obtained.
Regulations & IFRA
This ingredient is not restricted

