Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
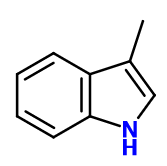
CAS N° : 83-34-1
-
EINECS number : 201-471-7
-
FEMA number : 3019
-
Density : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Base
-
Price Range : €€€€
-
Appearance : White solid
-
FLAVIS number : 14.004
-
JECFA number : 1304
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 266°C (à 255 mmHg)
-
Detection Threshold : 0,2 ppb (0,00000002%)
-
Molecular formula : C9H9N
-
Log P : 2,60
-
Molecular Weight : 131,17 g/mol
-
Fusion Point : 96°C
-
Flash Point : 132°C
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
Skatole is more powerful and greedy than Indole but less floral.
Stability :
This compound is well known for synthesizing Schiff bases by reacting espacially with aldehydes. These compounds can have an olfactive interest but are still a source of coloration in perfume concentrates and perfumes besides others. This is why this raw material is used is small quantities.
Uses in perfumery :
Skatole is used in small quantities in leather, woody notes, but less in floral notes than Indole. Brings an animalic facet to all types of accords.
Year of discovery :
1880
Isomerism :
Skatole does not have isomer used in perfumery.
Synthesis precursor :
Skatole is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Skatole is found naturally in some cheeses, among others, but is very little extracted for its use in perfumery. Synthetic skatole is the most used.
Synthesis route :
Skatole is part of the same family of molecules as Indole: the pyroles. These molecules can be synthesized in several ways. In the case of Skatole, a Fischer synthesis is adapted. This reaction consists in reacting phenylhydrazine with propanal, catalysed by a strong acid. Many other synthetic routes exist for this compound, such as the synthesis of Bischler-Möhlau, the Reissert or the Madelung synthesis among others.
Regulations & IFRA
This ingredient is not restricted

