Photo credits: ScenTree SAS
Terpineol
α,4-trimethyl-(1S)-3-cyclohexene-1-methanol ; α,α,4-trimethyl-(1R)-3-cyclohexene-1-methanol ; 1-methyl-4-(1-methylethylidene)-cyclohexanol ; p-Menth-1-en-8-ol ; 2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 8000-41-7
-
EINECS number : 202-680-6
-
FEMA number : 3045
-
Density : 0,93
-
Optical rotation : Donnée indisponible
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Donnée indisponible
-
Volatility : Head
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : 02.014
-
JECFA number : 366
Information on synthetic ingredients
-
Acid Value : Donnée indisponible
-
Boiling Point : 214–217°C (417–423 °F)
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C10H18O
-
Log P : 2,91
-
Molecular Weight : 154,25 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 89°C (192°F)
-
Vapor pressure : Donnée indisponible
Uses
Other comments :
Terpineol, along with menthol-l and carvone-l, is one of the three most important cyclic monoterpenes used in perfumery. Precursor to the synthesis of many other ingredients and present at least trace in most natural extracts, terpineol is essential in perfumery.
The quality presented here comes from a Upcycling program and is 100% renewable carbon.
Stability :
Data not available.
Uses in perfumery :
Besides the fact that terpineol is widely used for the synthesis of other olfactive ingredients, it can also be used as such in our formulas. Its refreshing pine-like odor is widely used in the detergent and home care industry as well as for creating olfactory reconstitutions and bases. It is often detected in chromatography, because this ingredient is found in a large number of essential oils.
Year of discovery :
Terpineol's structure determined in 1885 by Wallach, Tiemann and Semler.
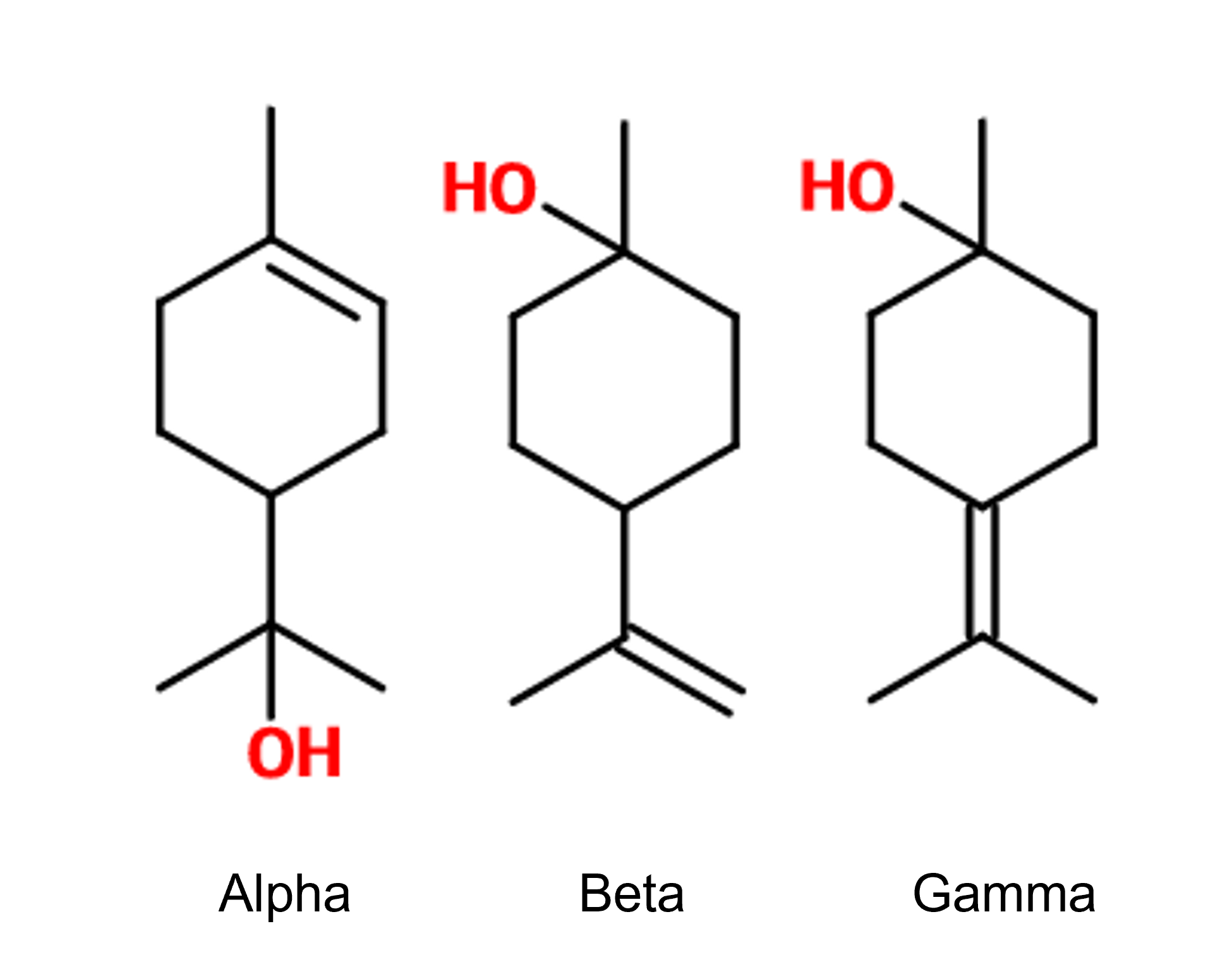
Isomerism :
Terpineol is a mixture of isomers. There are four of them. The alpha-terpineol ; beta-terpineol ; gamma-terpineol as well as terpinen-4-ol.
Synthesis precursor :
Terpineol is a precursor for the synthesis of a large number of olfactory compounds.
Natural availability :
Terpineol can be obtained from the essential oil of many plants by fractional distillation, such as Turpentine EO, Lavender EO, Wormwood EO, Cardamom EO or Clary Sage EO. It is therefore posible to have a natural quality. This ingredient is found, in large quantities or in trace, in a very large quantity of ingredients used in our fragrances. Some references identify more than 200 oils containing it.
Synthesis route :
There are several ways to obtain Terpineol. Either by isolation from a natural ingredient, or by synthesis. The most commonly used reaction mechanisms start with Turpentine EO, alpha-pinene or D-Limonene
Regulations & IFRA
This ingredient is not restricted

